Reaction rates and surface area
Simulation
Have you ever wondered why tablets dissolve faster in water when you crush them up first? Let's dive into the molecular level to understand what's happening.
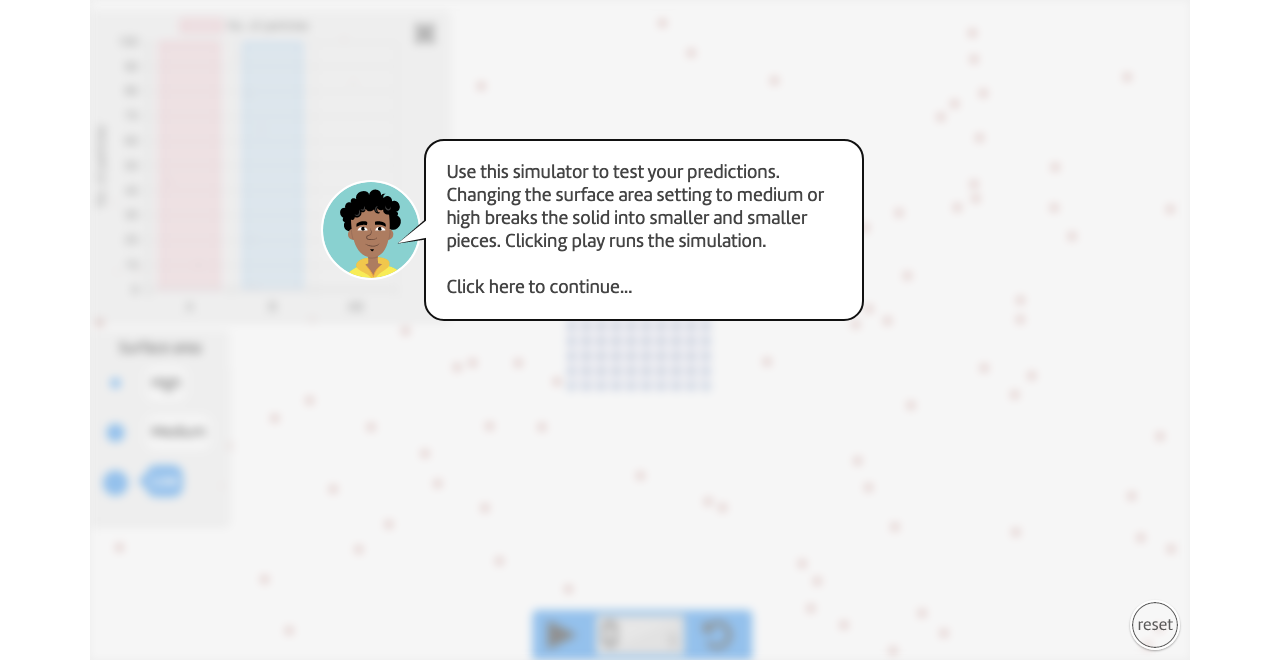
Instructions
Draw a four-column table in your notebook. Label the left-hand column "Time (s)", the next column "Number of particles – low surface area", then "Number of particles – medium surface area", then "Number of particles – high surface area".
Write "0", "5", "10", and "15" in the top four rows of your time column.
Run the simulation for "low surface area".
Pause the simulation at 5, 10, and 15 seconds.
Record the number of AB particles in your table.
Repeat steps 3-5 for medium and high surface areas.
Discussion questions
How does "high surface area" represent a crushed up tablet and "low surface area" represent a whole tablet?
Think of a real-life example of a liquid reacting with a solid, like in this simulation. What is better, a high or low surface area?
Details
NGSS
- DCI | PS1.B: The total number of each type of atom is conserved, and thus the mass does not change.
Learning Objectives
Understand the relationship between rates of reaction and surface area.
Concepts
Chemical reaction
Reaction rate
Surface area
More Chemistry simulations

Making plastics from atoms
Unleash your inner mad scientist and create plastics from atoms!
Making molecules and lattices from atoms
Grab your safety goggles, it's time to create some molecules!
Reaction rates and temperature
Vary temperature and measure the change in reaction rate.
Exothermic or endothermic?
Add molecules into the reaction, add energy, and see what happens!
