Reaction rates and temperature
Simulation
Intuitively we understand that the higher the temperature the faster a chemical reaction. But what's actually happening at a molecular level?
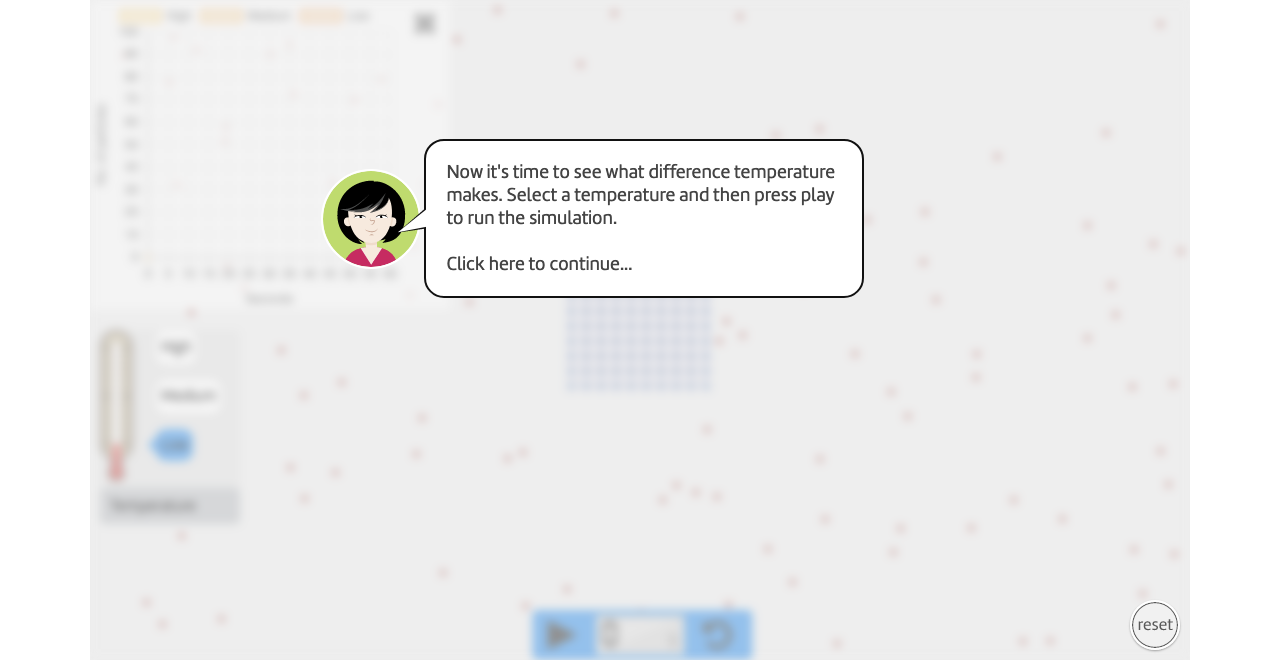
Instructions
Run the simulation for "low" temperature for 20 seconds.
Hit the pause button.
Change the temperature to "medium" temperature.
Run the simulation for another 20 seconds.
Hit the pause button.
Change the temperature to "high" temperature.
Run the simulation for another 20 seconds.
Discussion question
Using everyday language that you might use with your grandparents, explain to the person sitting next to you how temperature affects the rate of chemical reactions.
Details
NGSS
- DCI | PS1.B: The total number of each type of atom is conserved, and thus the mass does not change.
Learning Objectives
Understand the relationship between rates of reaction and temperature.
Concepts
Chemical reaction
Reaction rate
Temperature
More Chemistry simulations

Making plastics from atoms
Unleash your inner mad scientist and create plastics from atoms!
Making molecules and lattices from atoms
Grab your safety goggles, it's time to create some molecules!
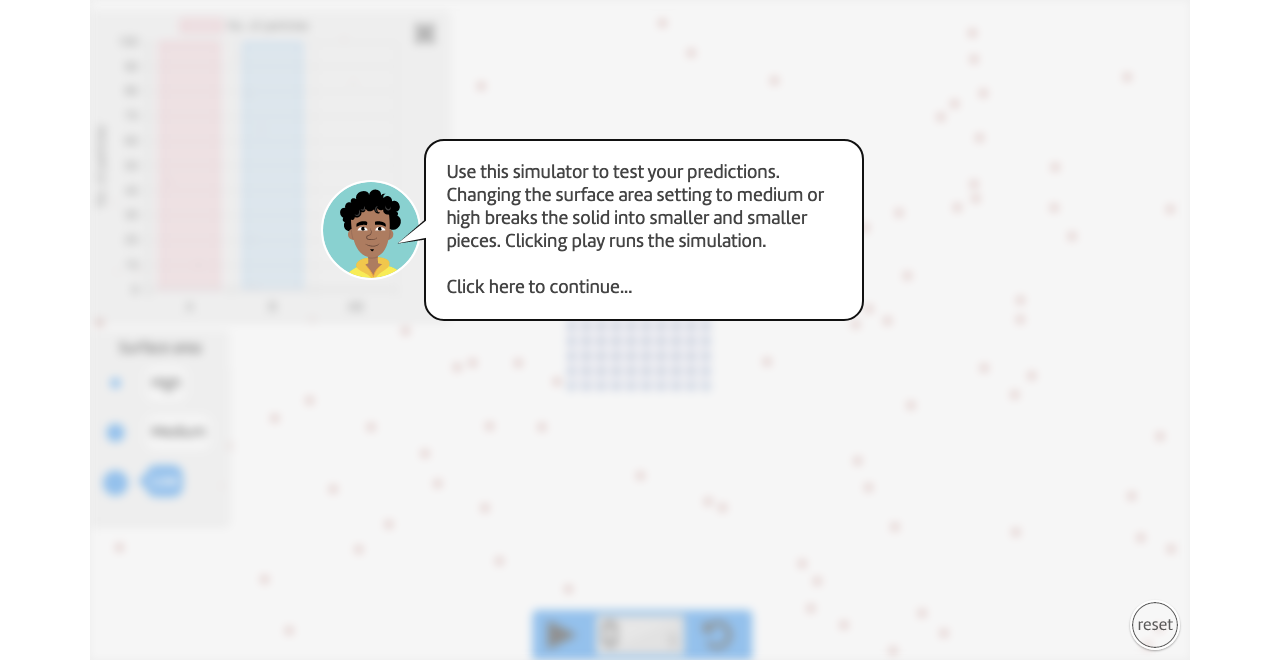
Reaction rates and surface area
Vary surface area and measure the change in reaction rate.
Exothermic or endothermic?
Add molecules into the reaction, add energy, and see what happens!
